Minerva Neurosciences Announces Study Results Demonstrating Bioequivalence of Phase 2b, Phase 3, and Planned Commercial Formulations of Roluperidone for Treatment of Negative Symptoms of Schizophrenia
“The results demonstrate bioequivalence in terms of exposure between the formulations used in our two late-stage Phase 2b and Phase 3 efficacy and safety trials with roluperidone and we believe that the data address certain FDA observations following the Company’s Type C meeting in November 2020,” said Dr.
The area under the curve to last detectable concentration (AUClast), the area under the curve extrapolated to infinity (AUCinf), and the maximum plasma concentration (Cmax) are the most commonly used plasma pharmacokinetic parameters to evaluate bioequivalence between various formulations.
For roluperidone, efficacy is mostly driven by plasma exposure of the drug (i.e., AUCs) whereas safety margins improve by reducing Cmax of the drug. Furthermore, as roluperidone is intended for chronic use and the assessed formulations are controlled release, AUCinf is the most relevant of the AUCs when single dose data are collected and used for determining bioequivalence.
In this study, the two most important objectives were to establish:
- The comparability under fasted condition of the 64 milligram (mg) tablet of the Phase 3 formulation of roluperidone compared to the 64 mg dose based on the administration of two 32 mg tablets of roluperidone used in the Phase 2b study, and
- The comparability under fasted condition of a 64 mg tablet of the planned commercial formulation of roluperidone compared to the 64 mg dose based on the administration of two 32 mg tablets of roluperidone used in the Phase 2b study.
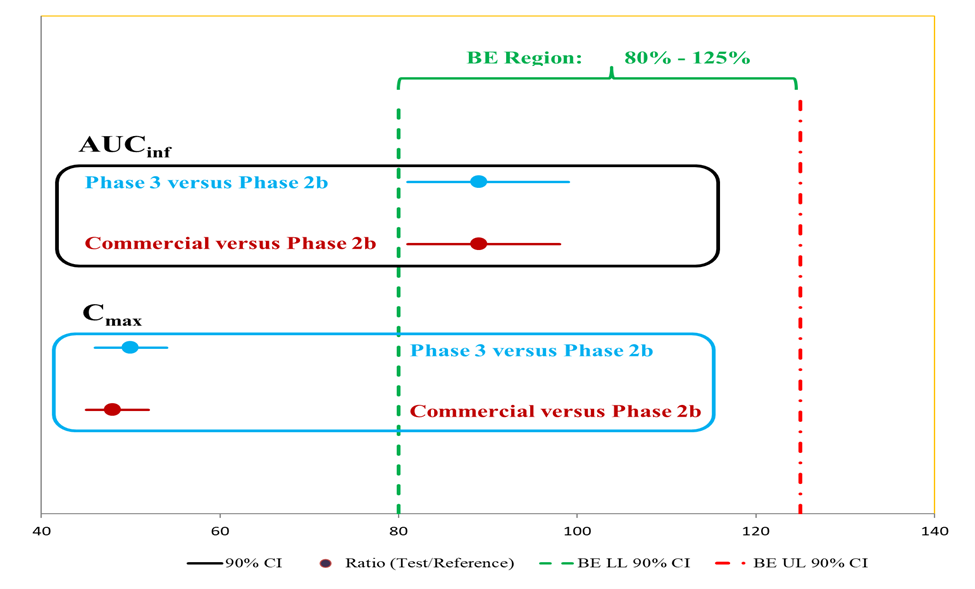
As presented in Figure 1 for both objectives, the AUCinf were bioequivalent, and the Cmax of the reformulated phase 3 and planned commercial formulations were reduced substantially compared to the Phase 2b formulation.
https://www.globenewswire.com/NewsRoom/AttachmentNg/b1fde1a5-9b71-4511-a5ed-ec76d67b364a
Figure 1: Comparisons of AUCinf and Cmax between Phase 3 and phase 2b formulations, and planned commercial and Phase 2b formulations: 90% CI = 90% Confidence Interval; Ratio (Test/Reference); BE LL 90% CI = Bioequivalence Lower Level 90% Confidence Interval cutoff; BE UL 90% CI = Bioequivalence Upper Level 90% Confidence Interval cutoff
The additional two objectives of the study were to establish:
- The comparability under fasted condition of the 64 mg formulation of the planned commercial tablets compared to that used in the Phase 3 for which the results show bioequivalence between the formulations in terms of AUCs and Cmax.
and
- The comparability of the 64 mg dose of the commercial formulation under fed condition compared to fasted condition for which the results show bioequivalence of both AUCinf and Cmax between the fed and fasted conditions.
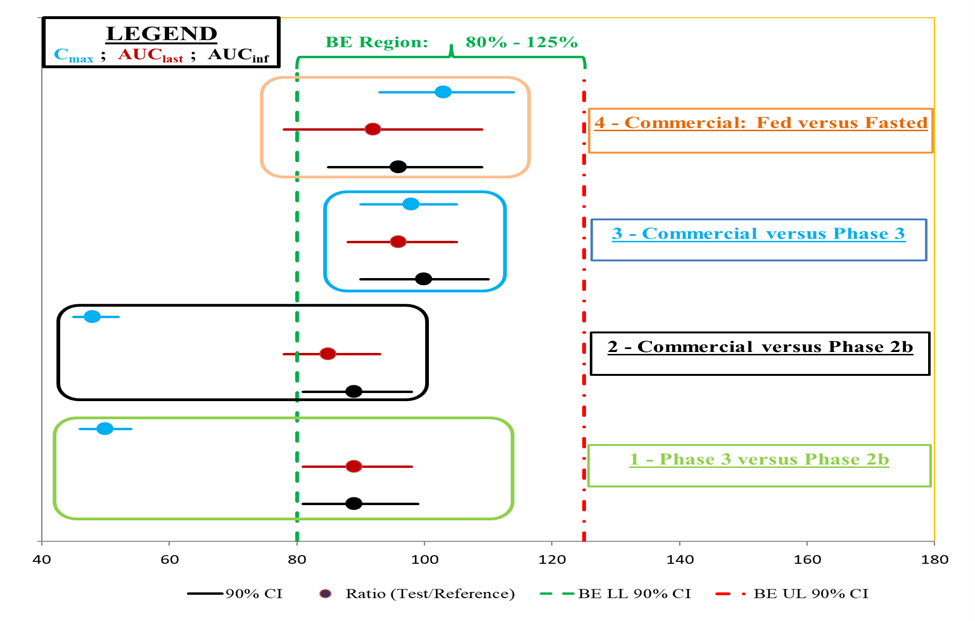
Figure 2 presents a summary of the four study objective results:
https://www.globenewswire.com/NewsRoom/AttachmentNg/626c9e24-4fde-43f4-9196-c8e3e9220971
Figure 2: The four study objectives are listed on the right across from the bioequivalence testing results for Cmax, AUClast, and AUCinf. Other symbols are similar to those presented in Figure 1 above.
Study description:
Subject screening in this study was initiated on
Of the 48 subjects randomized, 45 completed all study periods. Male subjects constituted 69% of the participants, and 75% of the subjects were white. Median age was 36 years, and all had negative SARS-CoV2 status at the beginning of the study and of every study period with the exception of 1 subject
About Schizophrenia and Negative Symptoms
Schizophrenia is a chronic, severe and debilitating type of mental illness characterized by distortions in thinking, perception, emotions, language, sense of self and behavior. Schizophrenia affects 20 million people worldwide. (
Negative symptoms can cause individuals with schizophrenia to withdraw from society, become disinterested or unable to complete tasks or feel pleasure. Negative symptoms are characterized by five constructs: blunted affect, alogia, avolition, anhedonia, and asociality (Marder and Galderisi, 2017).
Negative symptoms are the main cause of the poor functional outcome of patients suffering from schizophrenia (Harvey et al., 2020) and may also be one of the main reasons ultrahigh risk adolescents may develop full blown schizophrenia (Gomes and Grace, 2017). There are currently no treatments approved for negative symptoms of schizophrenia.
About
Forward-Looking Safe Harbor Statement
This press release contains forward-looking statements. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this press release, and involve certain risks and uncertainties. Forward-looking statements include, but are not limited to, statements herein with respect to the timing and scope of clinical trials and regulatory review and results and outcomes of such clinical trials, including the clinical development of roluperidone (MIN-101) for the treatment of negative symptoms of schizophrenia; the clinical and therapeutic potential of this compound, including its potential benefits in the treatment of negative symptoms of schizophrenia or any other indication; the timing and outcomes of future interactions with
For more information:
Investor inquiries:
CFO,
Email
Media Inquiries:
Principal,
Email

Figure 1
Comparisons of AUCinf and Cmax between Phase 3 and phase 2b formulations, and planned commercial and Phase 2b formulations: 90% CI = 90% Confidence Interval; Ratio (Test/Reference); BE LL 90% CI = Bioequivalence Lower Level 90% Confidence Interval cutoff; BE UL 90% CI = Bioequivalence Upper Level 90% Confidence Interval cutoff
Figure 2
The four study objectives are listed on the right across from the bioequivalence testing results for Cmax, AUClast, and AUCinf. Other symbols are similar to those presented in Figure 1 above.
Source: Minerva Neurosciences, Inc