UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 31, 2020
Minerva Neurosciences, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36517 | 26-0784194 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 1601 Trapelo Road Suite 286 Waltham, MA |
02451 | |
| (Address of principal executive offices) |
(Zip Code) |
(Registrant’s telephone number, including area code): (617) 600-7373
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common Stock, $0.0001 par value per share | NERV | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On March 31, 2020, Minerva Neurosciences, Inc. (the “Company”) hosted a webcast presentation in connection with a Key Opinion Leader (“KOL”) meeting on roluperidone and the treatment of negative symptoms of schizophrenia. A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in Item 7.01 of this report, including the information in the presentation attached as Exhibit 99.1 to this report, is furnished pursuant to Item 7.01 of Form 8-K and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section. Furthermore, the information in Item 7.01 of this report, including the information in the presentation attached as Exhibit 99.1 to this report, shall not be deemed to be incorporated by reference in the filings of the registrant under the Securities Act of 1933, as amended.
| Item 9.01 | Financial Statements and Exhibits |
| (d) | Exhibits |
| Exhibit No. | Description | |
| 99.1 | Company Presentation. | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| MINERVA NEUROSCIENCES, INC. | ||
| By: | /s/ Geoffrey Race | |
| Name: | Geoffrey Race | |
| Title: | Executive Vice President, Chief Financial Officer and Chief Business Officer | |
Date: April 1, 2020

Roluperidone: A potential novel mechanism to treat the negative symptoms of schizophrenia William Carpenter MD John Kane MD Steven Marder MD Ofer Agid MD Remy Luthringer PhD Hosted by Minerva Neurosciences (Nasdaq: NERV) Tuesday, March 31, 2020 Exhibit 99.1

All trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. Forward-Looking Statement Safe-Harbor This presentation contains forward-looking statements about Minerva Neurosciences which are subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this presentation, and involve certain risks and uncertainties. Forward-looking statements include, but are not limited to: the benefits, efficacy and safety of our new formulations; the potential of the diagnosis and treatment of negative symptoms of schizophrenia and other diseases; whether studies performed on analogs or backups of our compounds are a good predictor of the clinical efficacy of our compounds; statements with respect to the timing and results of future clinical milestones with roluperidone (MIN-101) and seltorexant (MIN-202), including the Phase 3 trial of roluperidone and the Phase 3 trials of seltorexant; statements regarding our ability to successfully develop and commercialize our therapeutic products; our expectations regarding approval for our products by the U.S. Food and Drug Administration or equivalent foreign regulatory agencies; estimates regarding the market potential for our products; and future performance. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking statements. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of factors including, without limitation, whether any of our therapeutic products will advance further in the clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether the results of future clinical trials of roluperidone, seltorexant and MIN-301, if any, will be consistent with the results of past clinical trials; whether roluperidone, seltorexant and MIN-301 will be successfully marketed if approved; whether our therapeutic product discovery and development efforts will be successful; our ability to achieve the results contemplated by our co-development agreements; the strength and enforceability of our intellectual property rights; competition from pharmaceutical and biotechnology companies; the development of and our ability to take advantage of the market for our therapeutic products; our ability to raise additional capital to fund our operations on terms acceptable to us; and general economic conditions. These and other potential risks and uncertainties that could cause actual results to differ from the results predicted are more fully detailed under the caption “Risk Factors” in our filings with the Securities and Exchange Commission, including our Quarterly Report on Form 10-K for the year ended December 31, 2019, filed with the Securities and Exchange Commission on March 9, 2020. Copies of reports filed with the SEC are posted on our website at www.minervaneurosciences.com. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we disclaim any obligation to update any forward-looking statements, except as required by law.

Agenda KOL discussion of the frequently asked questions about negative symptoms Roluperidone: an update

William Carpenter MD John Kane MD Steven Marder MD Ofer Agid MD Input from KOLs about the frequently asked questions regarding negative

Question 1: incidence of schizophrenia with negative symptoms How many patients with schizophrenia live in the US? How many are diagnosed and are getting treatment? How many have negative symptoms? How many do not have negative symptoms at all? Are there different sub-categories of patients among those manifesting negative symptoms? Which treatments are currently prescribed for negative symptoms? Are they efficacious?

Question 2: What is the right trial design for a novel drug to treat negative symptoms? How can we be sure we have a specific effect versus a pseudo-effect? Assuming that experienced investigators are well-trained in patient selection and use of scales, how likely it is that: Regional differences in results will nevertheless occur? Phase 3 will produce different results to the 2b study despite the fact that the designs are almost identical? Reference: Marder et al., 2020. Issues and Perspectives in Designing Clinical Trials for Negative Symptoms in Schizophrenia: Consensus Statements, Schizophrenia Bulletin. During the development of compounds targeting negative symptoms that have novel mechanisms of action, the evaluation of such compounds in a monotherapy design should also be considered in order to characterize the potential beneficial effects of such compounds in a condition without the potential confounding effects of background concomitant antipsychotic treatment. Also, if the experimental compound is more effective than placebo at treating psychosis it could result in greater reductions in negative symptoms that are secondary to positive symptoms. Similarly, an antipsychotic with less tendency to induce depression or extrapyramidal side effects (EPS) could result in greater improvements in secondary negative symptoms. In a prior manuscript from an ISCTM working group it was proposed that the comparison compound by an effective drug with minimal EPS. A commentary by Laughren and Levin from FDA pointed out that this design is not an optimal solution since it does not solve the problem of “pseudospecificity.” That is, it cannot rule out that improvement in negative symptoms is actually secondary to improvement in positive symptoms.

Question 3: Can you comment about the different attributes of the different NS scales which can be extracted from the PANSS scale? What are the specifics of the Marder score? N1. Blunted affect N2. Emotional withdrawal N3. Poor rapport N4. Passive/apathetic social withdrawal N5. Difficulty in abstract thinking N6. Lack of spontaneity N7. Stereotyped thinking N1. Blunted affect N2. Emotional withdrawal N3. Poor rapport N4. Passive/apathetic social withdrawal N6. Lack of spontaneity G7. Motor retardation G16. Active social avoidance Original Kay NS sub-scale Marder NS factor N1. Blunted Affect N2. Emotional Withdrawal N3. Poor Rapport N4. Passive/Apathetic Social Withdrawal N6. Lack of Spontaneity G5. Mannerisms and Posturing G7. Motor Retardation G8. Uncooperativeness G13. Disturbance of Volition G14. Poor Impulse Control White NS factor

Question 4: Can you comment on our current understanding of Negative Symptoms and cognitive impairment? How are the recent publications around roluperidone helping? References: Harvey et al., 2020. Effects of Roluperidone (MIN-101) on two dimensions of the negative symptoms factor score: Reduced emotional experience and reduced emotional expression. Schizophrenia Res. Strauss et al., 2020. Network Analysis Indicates That Avolition Is the Most Central Domain for the Successful Treatment of Negative Symptoms: Evidence From the Roluperidone Randomized Clinical Trial. Schizophrenia Bulletin. Keefe et al., 2017. Cognitive Effects of MIN-101 in Patients with Schizophrenia and Negative Symptoms: Results from a Randomized Controlled Trial. J Clin Psychiatry.

Question 5: Do currently available drugs have a beneficial effect which is specific for negative symptoms? Has the FDA ever approved a drug specifically targeting negative symptoms? Are antidepressants effective to treat primary, deficit, negative symptoms? Reference: Fusar-Poli et al., 2014. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophrenia Bulletin.

Question 6: Would a drug which improves function and/or is better tolerated enhance compliance? About 2/3 of the schizophrenic patients do not adhere to medication as prescribed 1,2 Although lack of insight is often mentioned, poor response and adverse effects are the main reasons for non-adherence 2,3 References: Czobor et al., 2015. Treatment adherence in schizophrenia: A patient level met-analysis of combined CATIE and EUFEST studies. Eur Neuropsychopharmacol. Barry et al., 2012. Schizophrenia. BMJ Clin Evid. Howes et al., 2017. Treatment Response and Resistance in Psychosis Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry.

Question 7: What is the current landscape of drugs under development for negative symptoms? How do you see the future research directions? “With myriad programs expecting near-term readouts and what we view as a favorable regulatory body for drug approvals in the mood disorder space, drug development in schizophrenia is undoubtedly a ‘hot’ and rejuvenated space.” Source: Neuro Navigator, Physician Survey Indicates Need for Novel Antipsychotics, March 19, 2020 Industry Report, Myles Minter, Ph.D. William Blair, used with permission

Question 8: How will roluperidone be prescribed in clinical practice? Can doses of anti-psychotics be reduced or even stopped in some sub-groups of patients? Reference: Huhn M et al., 2020. Reducing antipsychotic drugs in stable patients with chronic schizophrenia or schizoaffective disorders: a randomized controlled pilot trial. Eur Arch Psychiatry Clin Neurosci.

Question 9: Can Rx of negative symptoms benefit “at risk” youngsters and affect the rate of transition to schizophrenia ? Would first episode patients also benefit? References: Yung et al., 2019. Persistent negative symptoms in individuals at Ultra High Risk for psychosis. Schizophrenia Res. Addington & Heinssen, 2012. Prediction and prevention of psychosis in youth at clinical high risk. Annual review of clinical psychology

Question 10: Do Negative Symptoms manifest beyond schizophrenia? Are they comparable to the one seen in schizophrenia? 1.Schizophrenia 2. Schizoaffective Disorder 3. Schizophreniform Disorder 4. Schizotypal Personality Disorder 5. Schizoid Personality Disorder 6. Paranoid Personality Disorder 7. Avoidant Personality Disorder 8. Bipolar Disorder (I and II) 9. Major Depressive Disorder 10. Persistent Depressive Disorder (Dysthymia) 11. Premenstrual Dysphoric Disorder 12. Selective Mutism 13. Social Anxiety Disorder 14. Separation Anxiety Disorder 15. Reactive Attachment Disorder 16. Posttraumatic Stress Disorder 17. Depersonalization/Derealization Disorder 18. Autism Spectrum Disorder 19. Neurocognitive Disorders Reference: Strauss & Cohen, 2017. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophrenia Bulletin.

Roluperidone: An update Remy Luthringer PhD CEO & Executive Chairman Minerva Neurosciences

Roluperidone (MIN-101 C07): Pivotal phase 3 study to evaluate efficacy and safety in 515 schizophrenic patients with negative symptoms 4 week screening phase including washout (day -28 to day -1) 12 week double-blind treatment phase (day 1 to day 84) 40 week open label extension phase (day 85 to day 364) Placebo PO,QD MIN-101 32mg PO,QD MIN-101 64mg PO,QD Randomization Primary Endpoint Primary endpoint:Reduction in PANSS Negative Symptoms Factor Score (NSFS; Marder score) from baseline after 12 weeks administration Secondary endpoints: Personal and Social Performance scale (PSP), Clinical Global Impression of Severity (CGI-S), 40 weeks (9 months) open-label extension Number of patients: 515 patients randomized 1:1:1 Main inclusion criteria: DSM-5 schizophrenia diagnosis, Baseline score ≥ 20 on the 7 items PANSS negative score, Symptomatically stable and manifesting negative symptoms for 6 months as judged by the PI, Age 18-55 Powering Assumptions:90% powered and 40% drop-out rate Crossover MIN-101 32mg MIN-101 64mg

Enrollment completed – 515 patients randomized On track to report topline results from the 12-week, double-blind portion of the study in the second quarter of 2020 COVID-19 update: As the situation is continuously evolving, we are, with our CRO, in constant contact with the clinical sites to identify and mitigate risks including access to the clinical sites for patients and CRO personnel that may be impacted by travel restrictions, quarantines, etc. Minerva issued guidance to the clinical sites based on recent FDA and EMA guidelines allowing some protocol exceptions and ensuring that patients’ safety and access to study medication are given top priority Roluperidone (MIN-101C07): Pivotal Phase 3 trial update
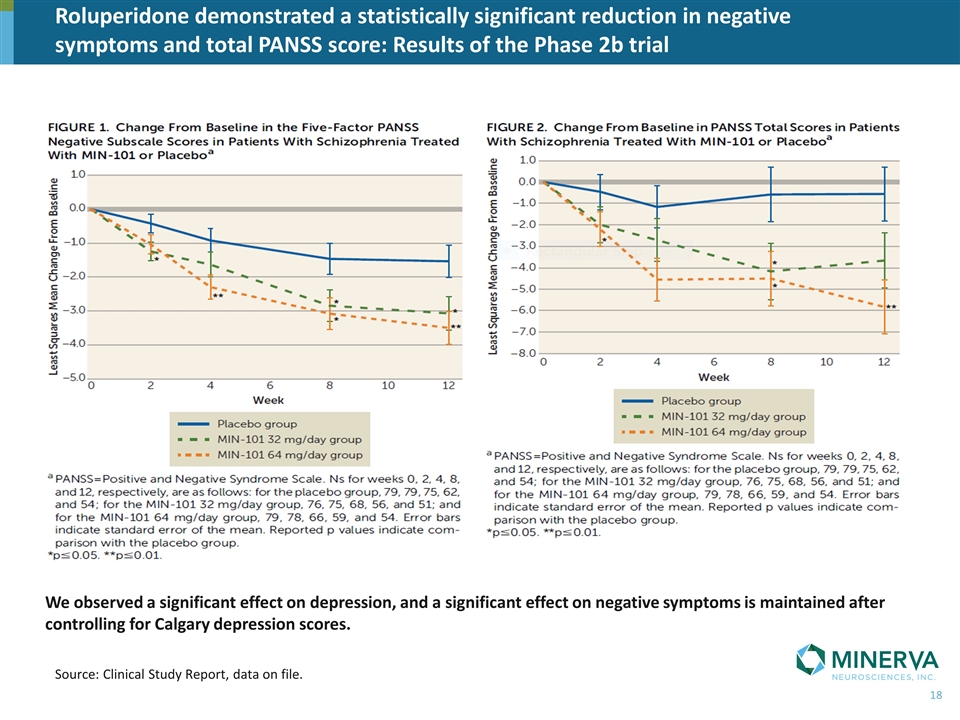
We observed a significant effect on depression, and a significant effect on negative symptoms is maintained after controlling for Calgary depression scores. Roluperidone demonstrated a statistically significant reduction in negative symptoms and total PANSS score: Results of the Phase 2b trial Source: Clinical Study Report, data on file.

Overall Phase 2b study results: Both doses of Roluperidone demonstrated statistically significant superiority to placebo in improving negative symptoms: Davidson et al., 2017; AJP Roluperidone was demonstrated to be superior on both PANSS-derived negative symptoms sub-scale as well on the BNSS, a scale specifically design to measure negative symptoms : Kirkpatrick et al., 2017; Schizophrenia Research Roluperidone 32 mg/day demonstrated statistically significant superiority to placebo in improving verbal fluency and motor token task, both measures of cognitive performance: Keefe et al., 2017; J Clin Psychiatry Roluperidone demonstrated statistically significant superiority to placebo in improving emotional experience and emotional expression: Harvey et al., 2020; Schizophrenia Research Roluperidone demonstrated statistically significant superiority to placebo in improving Avolition a central driver for overall negative symptoms: Network analysis: Strauss et al., 2020; Schizophrenia Bulletin Phase 2b results have been published in several peer-reviewed journals

How many patients diagnosed with schizophrenia could benefit from roluperidone if approved? 15%2,3 weighted-average 6-month relapse rate among patients with varying severity of negative symptoms 69% of patients have negative symptoms: ≈42%2,3 predominant/ prominent symptoms; ≈27%4 mild symptoms Prevalence of US adults with schizophrenia in treatment/yr: 0.53%1 Schizophrenia.com: 2.2 million patients in US Phase 3 enrolled population is representative of 780,000 patients in the US SZ=schizophrenia. 1.Wu et al. Psychol Medicine. 2006; 2. Millier et al. J Market Acc Health Policy. 2017; 3.Haro et al. Schizophr Research. 2015; 4. Nordstroem et al. J Social Psychiatry. 2017. Estimated prevalence of SZ (0.88%) 2.2 million US adults Treatment prevalence of SZ (0.53%) 1.3 million US adults Negative symptoms (69%) 0.9 million US adults Stable patients (85%): 0.78 million US adults

Decision Resources Group estimates roluperidone could account for 22% of schizophrenia market by 2027 LAI – long-acting injectable. Other drug classes include oral, LAI, short-acting IM injections, and other formulations of typical antipsychotics; short-acting IM injections and other formulations of atypical antipsychotics, Source: DRG’s Schizophrenia | Disease Landscape & Forecast, 2018. Last updated December 2018. 2017 G7 Sales: $6.5 billion 2027 G7 Sales: $7.8 billion

Paradigm shift? Best strategy to help patients with negative symptoms? Earlier-better? Severity of Illness Chronic Progression of Negative Symptoms & Cognitive Impairment Youth 0-18 Adult 18-40 Mature >40 Cognitive Symptoms Negative Symptoms Positive Symptoms Intermittent acute episodes of positive symptoms decline in frequency and severity Negative symptoms and cognitive impairment are evident at onset of illness and are lifelong debilitating symptoms All antipsychotics directly target dopamine (DA) receptors and have only shown efficacy against positive symptoms; none are indicated for negative symptoms or cognitive impairment Source of chart and captions: Minerva Corporate Presentation. Slide 7, January 2019 Source of statements: KOL Exploratories. January 9-10, 2018. Cello Heath Advantage Inc.

Additional Questions from Participants?
