Minerva Neurosciences Announces Results From Phase 3 Trial of Roluperidone (MIN-101) for Treatment of Negative Symptoms in Schizophrenia
- The 64 mg and 32 mg doses were not statistically significantly different from placebo at Week 12 on the primary endpoint, the PANSS Marder Negative Symptoms Factor Score (p ≤0.064 and 0.259, respectively), or the key secondary endpoint, the Personal and Social Performance Scale Total Score (p ≤0.021 and p ≤0.542, respectively)
- Roluperidone separated from placebo on both primary and key secondary endpoints at Weeks 4, 8 and 12
- Roluperidone was generally well tolerated with a safety profile comparable to placebo
|
||||||||||
Trial parameters and top-line results
In total, 515 patients were enrolled into the trial, and 513 patients received treatment and were included in the safety and Intent-To-Treat population. The trial was conducted in the
The results for both roluperidone doses versus placebo across both the primary and the key secondary endpoints to Week 12 were corrected for multiplicity using the truncated Hochberg procedure.
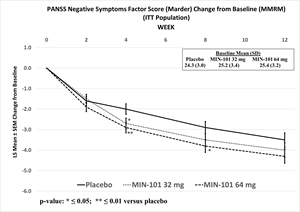
The primary objective of the trial was to evaluate the change from baseline to Week 12 of NSFS with 32 mg and 64 mg doses of roluperidone compared to placebo in patients diagnosed with schizophrenia presenting with moderate to severe negative symptoms. Neither the 32 mg nor 64 mg dose of roluperidone showed a statistically significant separation from placebo (32 mg: p ≤0.256, effect size [ES]=0.1; 64 mg: p ≤0.064, ES=0.2).
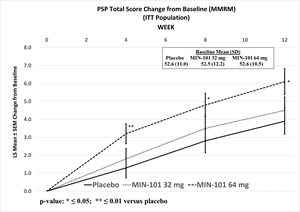
Furthermore, neither dose showed a statistically significant separation from placebo on the key secondary endpoint, the change from baseline to Week 12 in PSP (32 mg: p ≤0.542, ES=0.1; 64 mg: nominal p ≤0.021, ES=0.3).
Although limited inferences can be drawn from this data, unadjusted statistically significant separations from placebo were observed in NSFS at Week 4 for both doses (32 mg: nominal p ≤0.036, ES=0.2; 64 mg: nominal p ≤0.007, ES=0.3), and at Week 8 for the 64 mg dose (nominal p ≤0.027, ES=0.3), and the 64 mg dose was statistically significantly different from placebo as measured by change in PSP at all other assessment timepoints (Week 4, nominal p ≤0.005, ES=0.3; Week 8: nominal p ≤0.018, ES=0.3).
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/55de50fe-e501-4ef7-a1af-c2a72c8ddc54
https://www.globenewswire.com/NewsRoom/AttachmentNg/ad64a83c-09ed-4727-919a-cf137154cda1
Overall, subgroup analyses by region (
Roluperidone was generally well tolerated, and the incidences of patients who reported treatment‑emergent adverse events over the duration of 12 weeks of treatment were 37% for the 64 mg group, 42% for the 32 mg group, and 33% for placebo. Only 42 patients discontinued from the study due to adverse events, 16 (9%) in 64 mg arm, 18 (10%) in 32 mg arm, and 8 (5%) in placebo arm. Two treatment-unrelated deaths were reported in the 32 mg treatment arm.
“As someone who has spent his career studying everyday functioning in schizophrenia, I see disability as the most important treatment target for people with schizophrenia,” stated
“We are encouraged by the results obtained in this study which expand upon the outcome of the Phase 2b study that showed improvements in the primary endpoint and in multiple secondary endpoints,” said Dr.
The company will hold a webcast event on
About
Minerva’s proprietary compounds include: roluperidone (MIN-101), in clinical development for schizophrenia; seltorexant (MIN-202 or JNJ-42847922), in clinical development for insomnia and MDD; and MIN-301, in pre-clinical development for Parkinson’s disease. Minerva’s common stock is listed on the NASDAQ Global Market under the symbol “NERV.” For more information, please visit www.minervaneurosciences.com.
Forward-Looking Safe Harbor Statement
This press release contains forward-looking statements which are subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this press release, and involve certain risks and uncertainties. Forward-looking statements include statements herein with respect to the timing and scope of future clinical trials and results of clinical trials with roluperidone (MIN-101); the clinical and therapeutic potential of this compound; the timing and outcomes of future interactions with
Contact:
VP, Investor Relations/
Corp. Communications
(617) 600-7376
Source: Minerva Neurosciences, Inc